ABSTRACT
Objective: Fungal pulmonary infections are a significant complication in lung cancer, adversely affecting prognosis and treatment outcomes. This meta-analysis aimed to estimate the prevalence of chronic pulmonary aspergillosis (CPA) and Pneumocystis jirovecii pneumonia (PJP) in lung cancer patients and to identify associated clinical predictors. Methods: A systematic search of EBSCOhost, Embase, PubMed/MEDLINE, Scopus, and Web of Science retrieved 2,823 records, of which 7 studies were eligible (PROSPERO: CRD42024551104). Meta-analyses of proportions and dichotomous and continuous variables were performed using R (meta package) via Jamovi and RevMan 5, with statistical significance set at p<0.05. Results: Among 15,901 lung cancer patients, 177 had CPA and 135 had PJP. The pooled prevalence was 1% for CPA and 23% for PJP. CPA was significantly associated with male sex, smoking, COPD, interstitial lung disease, tuberculosis, and squamous cell carcinoma, and negatively associated with adenocarcinoma. CPA patients also had significantly lower BMI. Bilobectomy, radiotherapy, and concurrent chemoradiotherapy were additional risk factors for CPA. High-dose corticosteroid use (=20 mg/day) was significantly associated with PJP. Conclusion: CPA occurs in a clinically distinct subset of lung cancer patients with identifiable risk factors, while PJP appears to be strongly linked to immunosuppressive therapy. Improved screening strategies are warranted to mitigate the burden of these infections in vulnerable lung cancer populations.
Keywords:
lung cancer; fungal infection; pulmonary infection; aspergillosis; Pneumocystis jirovecii; pneumonia.
INTRODUCTION Lung cancer remains the leading cause of cancer-related mortality worldwide. It originates from epithelial cells of the respiratory tract and is broadly classified as small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC), the latter encompassing subtypes such as lung adenocarcinoma (LUAD) and squamous cell carcinoma (SCC).(1) Between 2001 and 2019, over 4 million U.S. patients were diagnosed,(2) and 2.2 million new cases were reported globally in 2020.(3) A 2023 meta-analysis estimated overall lung cancer mortality at 6–16%,(4) though survival plummets to 18.6% in cases of metastatic disease.(5)
Fungal pulmonary infections (FPIs) are underrecognized yet critical complications in lung cancer, with profound effects on morbidity, treatment outcomes, and survival. Cancer-related immunosuppression—caused by chemotherapy, radiation, and targeted therapies—predisposes patients to opportunistic fungi such as Aspergillus spp., Pneumocystis jirovecii, and Cryptococcus spp. These pathogens exploit therapy-induced immune dysfunction, structural lung damage, and impaired mucociliary clearance to establish invasive or chronic infections.(6–11) FPIs often mimic or aggravate cancer-related symptoms, leading to diagnostic delays and complex clinical management.(6,7,9–11)
Chronic pulmonary aspergillosis (CPA), a progressive infection caused by Aspergillus species, is particularly prevalent in this population. A Japanese multicenter study reported CPA-complicated lung cancer in patients undergoing anticancer treatment, correlating with poor prognostic factors such as squamous cell histology and low body mass index (BMI).(6) While CPA itself was not a direct cause of mortality, it led to treatment interruptions (e.g., pneumonitis) and a median overall survival of 14.57 months.(6) Similarly, a cross-sectional cohort detected Aspergillus colonization in 47.8% of newly diagnosed, non-neutropenic lung cancer patients, with A. niger as the dominant species. Notably, A. niger showed concentration-dependent cytotoxicity in human lung fibroblasts, suggesting a potential role in accelerating tissue damage and cancer progression.(7) Post-surgical NSCLC patients receiving trimodality therapy also exhibited elevated CPA risk, particularly those with prior adjuvant chemotherapy or radiation pneumonitis. (10) Localized CPA responded well to surgical or antifungal intervention, whereas disseminated disease was frequently fatal, underscoring the importance of early diagnosis.(10)
Pneumocystis jirovecii pneumonia (PJP) further complicates lung cancer management. A nested PCR study in Turkey detected P. jirovecii DNA in 66.7% of lung cancer patients—threefold higher than in non-cancer controls—with symptoms such as anorexia and weight loss strongly associated with colonization.(8) These findings highlight the importance of systematic screening in symptomatic patients. Moreover, analysis of exhaled breath condensate identified A. niger, A. ochraceus, or Penicillium spp. in 27.9% of lung cancer patients, but not in healthy controls,(11) suggesting environmental or host-related factors may predispose to FPIs.
Despite these insights, significant knowledge gaps remain. Most available evidence stems from small, retrospective studies,(6,9,10) limiting generalizability. FPIs—including CPA and PJP—are associated with prolonged hospitalization, treatment disruptions, and increased mortality, underscoring the need for greater clinical vigilance, routine screening in high-risk subgroups, and integrated management strategies. (6,7,10) The present systematic review synthesizes current evidence on the prevalence, clinical predictors, and treatment-associated risk factors of FPIs in lung cancer patients, aiming to inform optimized diagnostic and therapeutic approaches.
METHODS Review Question The objective of this systematic review and meta-analysis was to determine the prevalence of FPIs in lung cancer patients and to identify potential clinical factors associated with these infections. The study question was framed using the PEO structure, as follows:
Population (P): patients with lung cancer, including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) subtypes;
Exposure (E): pulmonary colonization by pathogenic fungal species;
Outcome (O): prevalence and clinical co-occurrence of FPIs—primarily CPA and PJP—in lung cancer patients.
The decision to focus on CPA and PJP as outcomes of interest was based on a pilot systematic search, which showed that these infections were the only ones consistently addressed as standalone topics in full-length observational studies. Other FPIs were predominantly described in isolated case reports.
Systematic Search Strategy A systematic literature search was conducted across five major databases: EBSCOhost, Embase, PubMed/MEDLINE, Scopus, and Web of Science. The predefined search strategy combined four primary keyword domains and their synonyms: fungal pulmonary infection, respiratory tract, lung cancer, and clinical outcome. Detailed PubMed/MEDLINE queries are provided in Supplementary Table S1, with corresponding strategies for EBSCOhost, Embase, Scopus, and Web of Science in Supplementary Tables S2–S5.
The review followed a registered protocol (PROSPERO ID: CRD42024551104); however, the present analysis specifically focused on FPIs as a targeted subset of the broader review. The search and reporting processes adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.(12)
Eligibility Criteria Studies were eligible for inclusion if they:
Investigated the association between clinical factors and fungal pulmonary infections (FPIs) in lung cancer patients;
Were observational in design, including prospective or retrospective cohorts, case-control studies, or cross-sectional studies, provided they reported data separately for lung cancer patients with and without FPIs;
Reported data on at least one of the following variables: demographics (age, sex, smoking history), comorbidities (e.g., cardiovascular disease, diabetes, underlying pulmonary disease), interventions (e.g., therapeutic regi-mens, surgical procedures), tumor histopathology, or cancer stage.
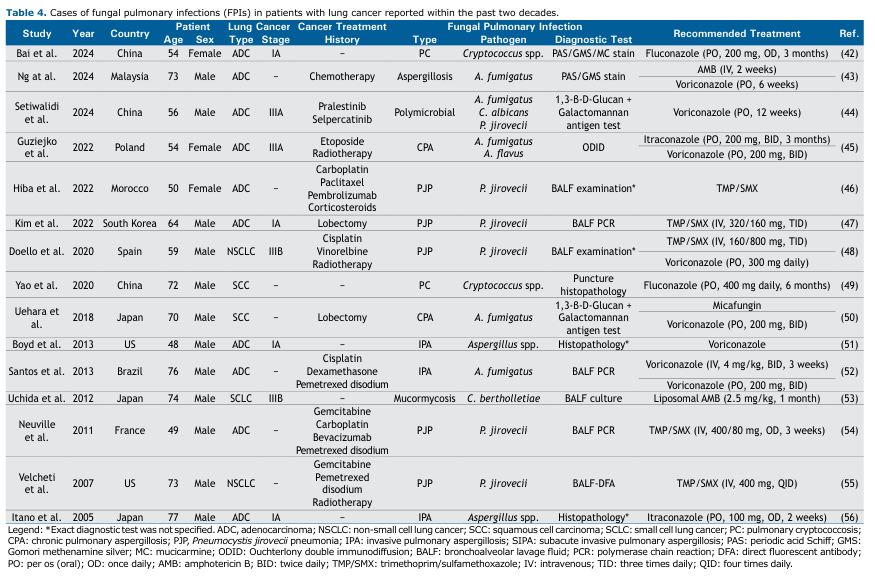
No restrictions were applied with regard to language or publication date. Records other than original research articles—including reviews, perspectives, editorials, and notes—as well as studies lacking the required data were excluded. Case reports were also excluded from the systematic review and meta-analysis in accordance with predefined criteria; however, a separate summary table of these case reports was compiled and included in the Discussion section to provide complementary insights into rare fungal infections and their clinical management.
Study Screening and Data Extraction Identified records were managed using Mendeley Desktop (version 1.19.8) (Mendeley, Elsevier, The Netherlands). Duplicate records were removed, and the studies were screened in two stages: (a) title and abstract screening to exclude irrelevant studies, and (b) full-text review to confirm eligibility. Data were extracted on study characteristics, effect measures, and relevant variables.
Risk of Bias Assessment The risk of bias in the included studies was assessed using the Risk Of Bias In Non-randomized Studies–of Interventions (ROBINS-I) tool,(13) and the results were visualized with the RobVis package (https://mcguinlu.shinyapps.io/robvis/) to enhance transparency.(14) ROBINS-I is the recommended instrument for evaluating bias in observational clinical studies of patient populations with defined conditions and treatment exposures,(13) making it suitable for the present review. The tool covers seven domains: (1) confounding factors; (2) participant selection; (3) classification of interventions; (4) deviations from intended interventions; (5) missing data; (6) measurement of outcomes; and (7) selection of the reported result.(13) Each domain was rated as having low, moderate, serious, or critical risk of bias.
Statistical Analysis A meta-analysis of proportions was conducted to estimate the prevalence of CPA and PJP in lung cancer patients using the meta package in RStudio (R version 4.2) under a random-effects model. Meta-analyses of dichotomous and continuous variables (clinical predictors) were performed separately for CPA and PJP using RevMan 5 (https://revman.cochrane.org/). A random-effects model was applied for variables exhibiting substantial heterogeneity (I² > 60%), while a fixed-effects model was used for those with moderate or low heterogeneity (I² ≤ 60%). All analyses used the restricted maximum-likelihood (REML) estimator, with statistical significance set at p<0.05. Heterogeneity was assessed with the I² statistic and its corresponding p-value. Results are presented in data tables and weighted forest plots. CPA and PJP were treated as distinct subgroups in all analyses. Only clinically relevant and statistically significant findings are shown as forest plots in the main text; complete sets of plots for all variables are available in the Supplementary Material.
RESULTS Systematic Search Supplementary Figure S1 presents the PRISMA 2020 flowchart of the systematic search. A total of 2,823 records were identified across EBSCOhost, Embase, PubMed/MEDLINE, Scopus, and Web of Science. After the removal of 91 duplicates, 2,732 records remained for title and abstract screening, of which 2,693 were excluded for not meeting the inclusion criteria. The full texts of the remaining 39 publications were reviewed, yielding 36 potentially eligible studies. Of these, 29 were excluded due to the absence of patient grouping based on the presence or absence of FPIs. Ultimately, 7 studies were included for assessment.
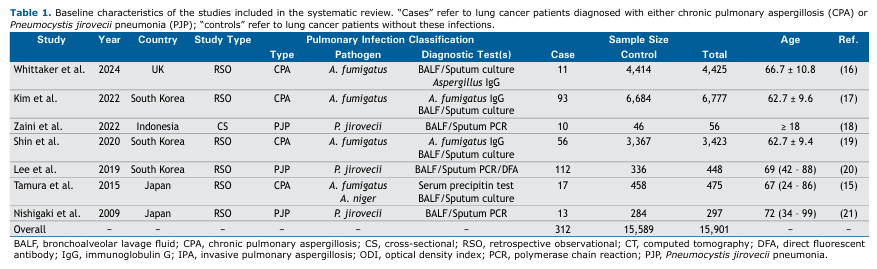
Table 1 summarizes the basic characteristics of the 7 included studies, which were published between 2009 and 2024. Most were observational studies conducted in Asian populations, with additional data from the UK, involving a total of 15,901 lung cancer patients, of whom 312 had concurrent FPIs: 177 CPA cases (4 studies) and 135 PJP cases (3 studies). Diagnoses were based on clinical signs and symptoms, supported by computed tomography (CT) imaging and sputum or bronchoalveolar lavage fluid (BALF) testing, including microbial culture and/or PCR. All CPA studies identified Aspergillus fumigatus as the causative strain, except Tamura et al.,(15) who also isolated Aspergillus niger.
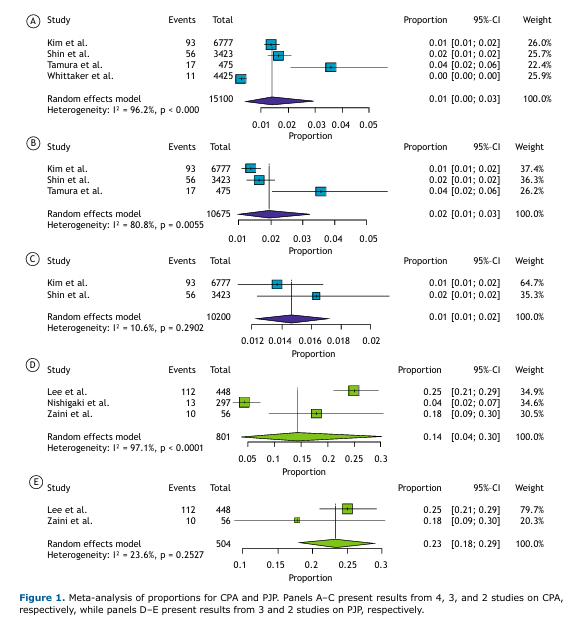
Prevalence of CPA and PJP in Lung Cancer Patients Meta-analyses of proportions for the CPA and PJP subgroups are presented in Figure 1. The initial analyses, which included all 4 studies on CPA and all 3 on PJP, showed substantial heterogeneity. In order to address this, studies contributing disproportionately to heterogeneity were sequentially excluded until acceptable heterogeneity levels were achieved. The final pooled prevalence estimates were 1% for CPA (95%CI: [0.01–0.02]; I² = 10.6%) based on a total sample size of 10,200 patients, and 23% for PJP (95%CI: [0.18–0.29]; I² = 23.6%) based on 504 patients.
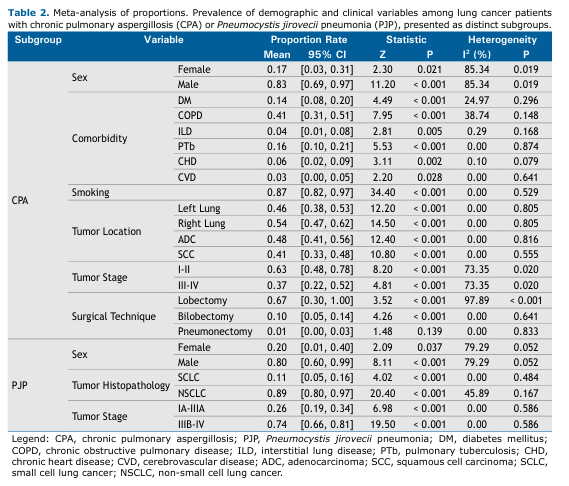
Prevalence of Demographics and Clinical Factors in CPA and PJP Subgroups of Lung Cancer Patients Table 2 presents the results of the meta-analysis of proportions for demographic and clinical factors among lung cancer patients with CPA and PJP. Corresponding forest plots are shown in Supplementary Figures S2–S25.
Among the CPA patients, males predominated, with a pooled mean prevalence of 83% (95%CI: [0.69–0.97]). Comorbidities were generally less common in this subpopulation, except for chronic obstructive pulmonary disease (COPD), which had a pooled mean prevalence of 41% (95%CI: [0.31–0.51]). A positive smoking history was highly prevalent (87%). Tumor localization and histopathology were largely comparable across patients, while early-stage disease (stages I–II) was more frequent than advanced-stage disease (stages III–IV) (63% vs. 37%, respectively). Surgical management in this subgroup was predominantly lobectomy, with a pooled mean prevalence of 67% (95%CI: [0.30–1.00]). These findings are summarized in Table 2.
Studies involving lung cancer patients with PJP generally reported fewer clinical variables, resulting in a narrower breadth of findings. Similar to CPA, males predominated among PJP patients, with a pooled mean prevalence of 80% (95%CI: [0.60–0.99]; Table 2). Regarding tumor histopathology, NSCLC, including adenocarcinoma (ADC) and squamous cell carcinoma (SCC), was far more frequent than SCLC (89% vs. 11%, respectively). In contrast to CPA, advanced disease was found to be more prevalent in this subgroup, with stage IIIB–IV tumors accounting for 74% of cases.
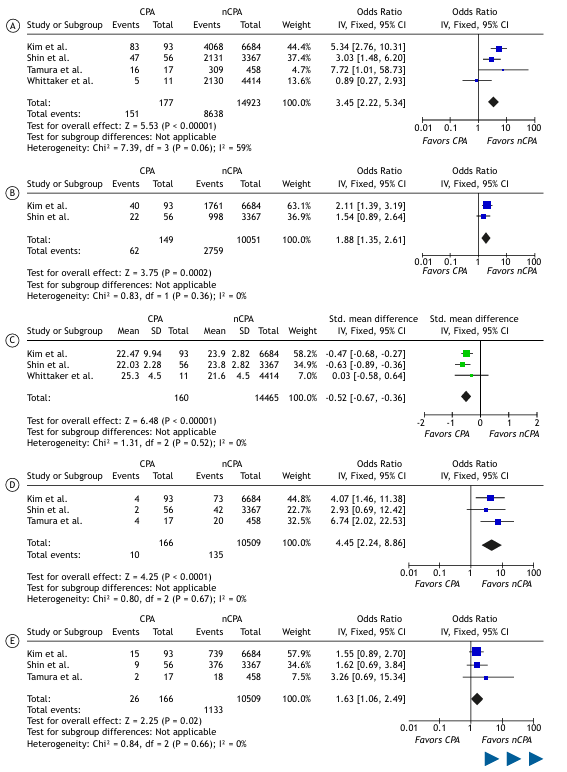
Meta-Analysis of Clinical Predictors of CPA and PJP in Lung Cancer Patients A meta-analysis of dichotomous and continuous outcomes comparing CPA vs. non-CPA and PJP vs. non-PJP lung cancer patients is presented in Table 3, with statistically significant findings visualized as forest plots in Figure 2.
Compared with non-CPA patients, CPA patients had significantly higher odds of male sex (OR: 3.11, 95%CI: [1.38–6.98]), smoking (OR: 3.92, 95%CI: [2.56–6.00]), COPD (OR: 2.22, 95%CI: [1.07–4.56]), interstitial lung disease (OR: 4.45, 95%CI: [2.23–8.86]), pulmonary tuberculosis (OR: 1.63, 95%CI: [1.06–2.49]), and squamous cell carcinoma (OR: 2.20, 95%CI: [1.60–2.98]). Conversely, adenocarcinoma was associated with significantly lower odds in CPA patients (OR: 0.41, 95%CI: [0.30–0.55]).
For body mass index (BMI), a continuous variable, the meta-analysis yielded a statistically significant standardized mean difference (SMD) of -0.52 (95%CI: [-0.67 to -0.36]), indicating lower mean BMI in CPA patients compared to non-CPA patients. Heterogeneity was generally low for most statistically significant results, except for male sex and COPD, which showed moderately high levels of heterogeneity (I² > 60%).
Table 4 summarizes the results of the meta-analysis of demographic and clinical variables in PJP vs. non-PJP patients. Corresponding forest plots are provided in Supplementary Figures S26–S31. Unlike the CPA/non-CPA analysis, no clinical variables differed significantly between PJP and non-PJP patients. Odds ratios for sex (male/female), tumor histopathology (SCLC/NSCLC), and tumor stage (early/late) were broadly comparable, underscoring the need for further clinical investigations in this particular subgroup.
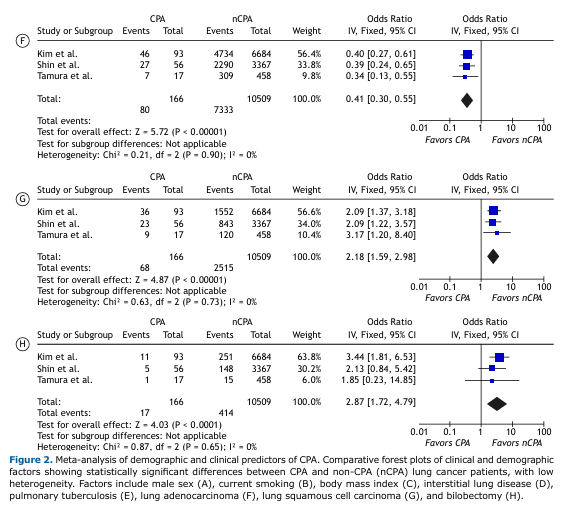
Treatment-Associated Risk of CPA in Lung Cancer Patients As shown in Figure 3, chemotherapy was not significantly associated with CPA in patients with lung cancer (OR: 1.29; 95%CI: [0.79–2.09]). In contrast, chest radiotherapy showed a significant association with CPA (p<0.001), with a pooled OR of 3.78 (95%CI: [2.14–6.68]) and negligible heterogeneity (I² = 0%). Concurrent chemoradiotherapy (CCRT) also showed a significant association (p=0.001), with a pooled OR of 4.06 (95%CI: [1.75–9.42]), though with substantial heterogeneity (I² = 75%).
Bilobectomy—defined as the surgical removal of two tumor-bearing lobes—emerged as a significant predictor of CPA, with a pooled OR of 2.87 (95%CI: [1.72–4.79]) and low heterogeneity (I² = 0%). Other surgical procedures, including lobectomy (single-lobe resection) and pneumonectomy, were not significantly associated with CPA (Table 3).
Collectively, these findings suggest that radiotherapy (alone or combined with chemotherapy) and bilobectomy may represent important risk factors for CPA development in lung cancer patients.
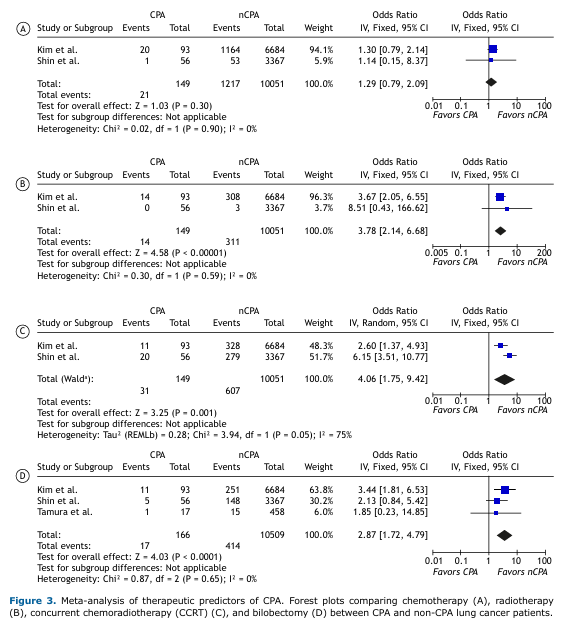
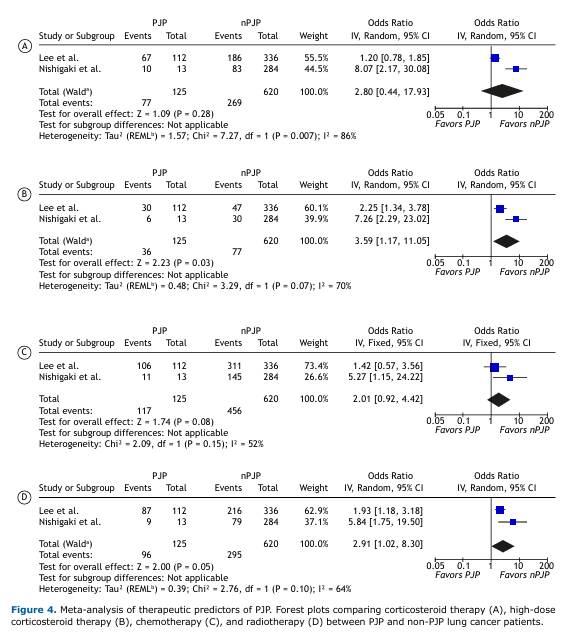
Treatment-Associated Risk of PJP in Lung Cancer Patients Overall, corticosteroid therapy was not significantly associated with PJP in lung cancer patients (OR: 2.80; 95%CI: [0.44–17.39]; Figure 4). However, high-dose corticosteroid use—defined as a daily dose of ≥20 mg—was significantly associated with increased odds of PJP (p=0.03), with a pooled OR of 3.59 (95%CI: [1.17–11.05]) and moderately high heterogeneity (I² = 70%).
Consistent with the CPA findings, chemotherapy was not significantly associated with PJP (p=0.08). Radiotherapy, on the other hand, showed a borderline significant association with PJP (p=0.05), with a pooled OR of 2.91 (95%CI: [1.02–8.30]) and moderate heterogeneity (I² = 64%).
Risk of Bias Assessment The risk of bias in the included studies was assessed using the ROBINS-I tool. As shown in Supplementary Figure S32, most studies exhibited low risk of bias across the majority of domains. The main concerns were related to confounding factors (D1) and participant selection (D2), where several studies were judged to have moderate risk. Key confounders included age, sex, and smoking history between lung cancer patients with and without FPIs.
Participant selection was deemed to confer serious risk of bias in the study by Lee et al.,(20) which recruited patients with confirmed PJP and compared them with a cohort of lung cancer patients without PJP. Furthermore, the study by Zaini et al.(18) had missing information in certain domains, particularly regarding deviations from intended interventions and classification of interventions, mostly involving surgical procedures and therapeutic regimens in PJP and non-PJP groups. Overall, most studies were considered to have a moderate risk of bias.
DISCUSSION This systematic review and meta-analysis evaluated the prevalence of fungal pulmonary infections (CPA and PJP) in 15,901 lung cancer patients across seven studies, identifying 312 cases. Key clinical associations were also explored to support risk stratification and management.
Prevalence and Clinical Context After excluding heterogeneous studies, the pooled prevalence rates were 1% for CPA and 23% for PJP, indicating a higher frequency of PJP. The clinical association between PJP and immunocompromised states(22) contrasts with CPA’s association with structural lung damage,(23) which may partly explain the lower prevalence of CPA, despite the widespread use of chemotherapy in lung cancer patients.(24) A Chinese study reported fungal infections in 28.7% of patients based on sputum cultures, with a higher proportion having a history of radiotherapy (31.3% vs. 18.5%).(25) Japanese data showed 31% fungal PCR positivity in lung cancer patients, attributed largely to corticosteroid use.(26) Notably, PJP can arise without prior colonization, as observed in four cases without preceding fungal detection.(27) Emerging antifungal resistance, such as voriconazole resistance in 42.4% of Aspergillus isolates in Indonesia,(28) underscores the clinical relevance of FPIs despite the modest 1% prevalence of CPA reported in this meta-analysis.
Clinical Predictors of CPA Male sex, COPD, interstitial lung disease (ILD), and SCC histology were positively associated with CPA, while lower BMI showed an inverse correlation. A 2024 cohort study linked lung cancer to CPA, with a hazard ratio of 8.51.(29) Similarly, a nationwide Japanese observational study identified lung cancer, COPD, and ILD as major risk factors for CPA,(30) consistent with our findings. A large-scale French analysis of 17,290 CPA cases over a 10-year period further corroborated these associations.(31) In addition, a Spanish study reported a 9.5-fold increase in mortality among CPA patients with a history of lung cancer.(32) SCC histology and BMI <18.5 kg/m² predicted poorer survival in CPA patients,(6) aligning with our meta-analysis, showing higher odds of SCC and lower BMI in CPA cases. The clinical relevance of low BMI is further supported by an 11-year retrospective study from Brazil involving 91 CPA patients, which found a predominance of underweight individuals, reinforcing the association between low BMI and CPA susceptibility.(33) Notably, a large-scale cohort study of 7,021 patients with advanced NSCLC showed improved overall survival in obese patients receiving chemotherapy or immunotherapy compared with those of normal BMI,(34) further highlighting the prognostic implications of body weight in lung cancer populations.
Treatment-Related Associations Radiotherapy and CCRT were significantly associated with CPA. Chest radiotherapy, particularly when combined with chemotherapy, increases the risk of fungal infection, with the highest incidence occurring within three months of treatment initiation.(35) CCRT is especially linked to aspergillosis, with susceptibility peaking during the first three months of treatment. In a cohort of 4,450 patients, fungal infections were reported in 15.9% post-radiotherapy, with markedly higher rates in CCRT patients (60.5% vs. 39.5%). (36) These findings are consistent with our meta-analysis, which showed higher odds of CPA in lung cancer patients with prior CCRT exposure compared to those receiving radiotherapy alone (4.06 vs. 3.78), suggesting that CCRT confers an added risk. A two-year follow-up of 1,872 lung cancer patients undergoing radiotherapy identified CPA in 24 out of 54 cases (44.4%) of chronic pulmonary infections, establishing CPA as the predominant type in this setting.(37) Consistently, a two-decade retrospective survey in Japan of 187 NSCLC patients receiving postoperative CCRT reported CPA in 6 cases (3.2%). (10) This prevalence is approximately three times higher than the 1% weighted mean observed in our meta-analysis, underscoring the contributory role of CCRT in CPA development. The increased odds of CPA following radiotherapy may be partly explained by radiation-induced pneumonitis,(38) compounded by immunosuppression associated with CCRT, further facilitating fungal colonization and the occurrence of CPA.(10)
PJP and Corticosteroid Use High-dose corticosteroids (≥20 mg/day) were strongly associated with PJP, whereas lower dosages showed non-significant trends. The association between corticosteroid therapy and PJP is well-established in the literature.(39) Our analysis revealed a pooled mean OR of 2.80 (95%CI: [0.44–17.93]; Figure 4) for PJP in patients with a history of corticosteroid treatment, irrespective of dosage; however, this association did not reach statistical significance. In contrast, daily doses ≥20 mg were significantly associated with PJP. This finding is clinically relevant, as demonstrated by the 2024 PCP-MULTI group study, which reported that among 66 solid tumor patients with PJP, 44 (66.7%) were receiving corticosteroids at daily doses ≥40 mg at the time of infection. High-dose corticosteroid use was linked to significantly lower survival.(40) Collectively, these data support a dose-dependent risk of PJP, highlighting the importance of prophylaxis in patients receiving high-dose corticosteroid therapy.(41)
Potential Implications of Observed Heterogeneity Several instances of elevated heterogeneity were observed across our analyses, particularly in the meta-analyses of proportions. Initial pooled prevalence estimates for CPA and PJP showed substantial heterogeneity (I² > 90%), which was mitigated through subgroup analyses and stepwise exclusion of outlier studies. Residual high heterogeneity was mainly confined to descriptive variables, such as sex distribution and the proportion of lobectomy among CPA patients. While informative, these were not central to our clinical interpretations.
The core of our findings lies in the meta-analyses of binomial outcomes for clinical predictors, where heterogeneity was generally low to moderate. Exceptions include COPD in CPA patients (I² = 75%) and tumor stage (I/II vs. III/IV), the latter retaining high heterogeneity (I² = 90%) despite post-hoc harmonization of stage definitions across a limited number of studies. Corticosteroid use in PJP patients also showed considerable heterogeneity (I² = 86%) in the overall analysis, though the association was not statistically significant (p=0.28). In contrast, high-dose corticosteroid use demonstrated a significant association with moderate heterogeneity (I² = 70%). These elevated values likely reflect the small number of available studies and the geographical homogeneity of included cohorts, the majority of which were conducted in Asia. Accordingly, we emphasize results with lower heterogeneity, which we consider more reliable and generalizable.
Limitations and Generalizability to Diverse Populations Our analysis is largely based on retrospective observational studies from Asia, reflecting the limited research on FPIs in lung cancer patients elsewhere. Isolated case reports from Europe, North America, Africa, and South America can be found in the literature. Table 4 provides a summary of pertinent case reports published since 2005, documenting the co-occurrence of pulmonary infections and lung cancer. These reports—spanning diverse histologies (ADC, NSCLC), stages (IA–IIIB), and treatments (platinum regimens, radiotherapy, immunotherapy, corticosteroids)—lack uniform denominators and standardized diagnostic criteria, undermining prevalence estimates outside Asia.
In broader CPA cohorts, 1 year mortality ranges from 7% to 32%, and 5 year mortality from 38% to 52%, with pulmonary cavitation representing the key risk factor and CT imaging plus Aspergillus IgG serology remaining central to diagnosis and management. (57) Similarly, non HIV PJP occurs predominantly in immunocompromised hosts, with malignancy present in up to 46% of cases and systemic glucocorticoids in up to 76%, though without a clearly defined dose threshold.(58) Our identification of high dose corticosteroids (≥20 mg/day) as a predictor for PJP therefore offers novel, dose specific insight. Nonetheless, in the absence of prospective cohorts from non Asian regions applying consistent methodology, the external validity of our pooled prevalence and risk estimates remains constrained, underscoring the need for multinational studies with harmonized diagnostic and treatment protocols.
Assessment of Publication Bias A formal assessment of publication bias was carried out with caution, as the included outcomes involved <10 studies, which is commonly considered the minimum threshold for meaningful evaluation using funnel plots or Egger’s test.(59) Funnel plots for CPA and PJP studies are presented in Supplementary Figures S31–S33. The funnel plot for all CPA studies showed asymmetry, with the study by Whittaker et al.(16) identified as an outlier. Upon exclusion of this study, Egger’s regression yielded a Z score of 1.69 (p=0.090), indicating no significant asymmetry or publication bias. Egger’s regression for PJP studies produced a Z score of 0.664 (p=0.507), similarly suggesting no evidence of asymmetry or publication bias. Since the Whittaker et al.(16) study was not included in most clinical predictor and treatment-related meta-analyses due to insufficient data (Figures 3–4), we can conclude that publication bias is unlikely to have substantially influenced our findings.
Clinical Remarks and Practical Considerations In routine practice, FPIs in lung cancer are investigated only when clinical or radiologic findings—such as new infiltrates, persistent fevers, or nodular lesions—raise suspicion, rather than through universal screening.(57,58) For PJP, current guidelines recommend initiating prophylaxis once patients receive the equivalent of ≥20 mg prednisone daily for ≥4 weeks or,(60) according to the 2022 European Alliance of Associations for Rheumatology (EULAR) update, ≥15 mg daily for ≥2 weeks.(57) Trimethoprim sulfamethoxazole (TMP/SMX) remains the first line regimen, with dosing adjusted for renal function and desensitization protocols available for sulfa allergic patients. Its efficacy is well supported, including in a recent risk-benefit analysis of primary prophylaxis against PJP involving 419 patients receiving TMP/SMX.(61) Within lung cancer-associated case reports (Table 4), TMP/SMX consistently appears as the predominant treatment modality for PJP, reinforcing its central role. By contrast, CPA typically arises from structural lung damage—such as post radiotherapy pneumonitis or bilobectomy—and routine antifungal prophylaxis is neither standard nor practical given concerns about toxicity, drug interactions, and cost. (57) Instead, our findings suggest a risk stratified surveillance approach: patients undergoing high risk interventions, including bilateral lung resections or CCRT, may benefit from scheduled chest imaging or serum biomarkers (e.g., galactomannan, Aspergillus PCR; see Table 1) in the months following treatment to enable earlier CPA detection. Together, these observations support targeted PJP prophylaxis and individualized CPA monitoring strategies to optimize fungal infection management in lung cancer care.
This systematic review and meta-analysis underscores the prevalence and clinical relevance of fungal pulmonary infections (FPIs) in lung cancer patients, focusing on chronic pulmonary aspergillosis (CPA) and Pneumocystis jirovecii pneumonia (PJP). PJP emerged as the predominant infection, with a pooled prevalence of 23% compared to 1% for CPA, reflecting their distinct pathogenic mechanisms—immunosuppression for PJP versus structural lung damage for CPA. Key clinical predictors of CPA included male sex, coexisting COPD or interstitial lung disease, squamous cell carcinoma (SCC) histology, low body mass index (BMI), and prior radiotherapy or chemoradiotherapy. The inverse association between BMI and CPA risk highlights the contribution of nutritional status. For PJP, corticosteroid use was the main risk factor, with daily doses ≥20 mg significantly increasing the risk of infection, reinforcing the need for dose-aware prophylactic strategies.
These findings support a risk-based approach to screening and prophylaxis, emphasizing multidisciplinary collaboration among oncologists, pulmonologists, and infectious disease specialists. Treatment decisions, particularly those involving corticosteroids and radiotherapy, should be carefully balanced against infection risk. Future research should aim to develop validated risk models and evaluate targeted prophylaxis, optimal therapeutic regimens, and long-term outcomes. FPIs represent a clinically significant complication in lung cancer, and tailoring management to their distinct risk profiles may improve prevention and patient care.
ACKNOWLEDGMENTS This systematic review and meta-analysis is part of a research project (No. 402000212) registered with the Systems Biology and Poisonings Institute at Baqiyatallah University of Medical Sciences, Tehran, Iran. The study protocol was reviewed and approved by the Research Ethics Committee of Baqiyatallah University of Medical Sciences (approval ID: IR.BMSU.BLC.1402.069). We wish to thank all our colleagues at Baqiyatallah University of Medical Sciences for their valuable support.
AUTHOR CONTRIBUTIONS MS participated in the study conceptualization, data curation, formal analysis, investigation (lead), methodology, writing—original draft (lead), and writing—review & editing. MG participated in the study conceptualization, data curation, formal analysis, investigation, methodology (supporting), and project administration and supervision (supporting). MA participated in the study conceptualization, data curation (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), project administration and supervision (lead), and writing—review & editing.
REFERENCES 1. Shah A, Hunter-Smith D. Lung Cancer. In: Paulman PM, Taylor RB, Paulman AA, Nasir LS (eds.) Fam. Med., Cham: Springer International Publishing; 2022, p. 1203–10. https://doi.org/10.1007/978-3-030-54441-6_92.
2. Fu Y, Liu J, Chen Y, Liu Z, Xia H, Xu H. Gender disparities in lung cancer incidence in the United States during 2001–2019. Sci Rep. 2023;13:12581. https://doi.org/10.1038/s41598-023-39440-8.
3. Zhang Y, Vaccarella S, Morgan E, Li M, Etxeberria J, Chokunonga E, et al. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol. 2023;24:1206–18. https://doi.org/10.1016/S1470-2045(23)00444-8.
4. Tesfaw LM, Dessie ZG, Mekonnen Fenta H. Lung cancer mortality and associated predictors: systematic review using 32 scientific research findings. Front Oncol 2023;13:1308897. https://doi.org/10.3389/fonc.2023.1308897.
5. Santorsola M, Di Lauro V, Nasti G, Caraglia M, Capuozzo M, Perri F, et al. Tumour Burden Reporting in Phase III Clinical Trials of Metastatic Lung, Breast, and Colorectal Cancers: A Systematic Review. Cancers (Basel). 2022;14:3262. https://doi.org/10.3390/cancers14133262.
6. Morimoto K, Hamashima R, Yamada T, Yokoyama T, Kobayashi T, Tsuyuguchi K, et al. Clinical significance of chronic pulmonary aspergillosis in lung cancer patients undergoing anticancer drug therapy. Thorac Cancer. 2024;15:1882–8. https://doi.org/10.1111/1759-7714.15416.
7. Esmaeel HM, Mohamed SS, Alhewaig AA, Aboul-Nasr MB. Pulmonary Aspergillosis in Naïve Non-neutropenic Lung Cancer Patients. Curr Respir Med Rev. 2023;19:314–22. https://doi.org/10.2174/1573398x19666230816091304.
8. Halidi AG, Ölçen M, Gürbüz E, Ekici A, Aydemir S, Yılmaz H. Investigation of Pneumocystis jirovecii in Lung Cancer Patients with the Nested PCR Method. Turkiye Parazitoloji Derg. 2022;46:276–80. https://doi.org/10.4274/tpd.galenos.2022.44153.
9. Huang J, Lan C, Li H, Chen S, Lin Q, Weng H. Concomitant lung adenocarcinoma and pulmonary cryptococcosis confirmed by pathologic examinations. Medicine (Baltimore). 2019;98:e18316. https://doi.org/10.1097/MD.0000000000018316.
10. Sugimoto S, Soh J, Suzawa K, Miyoshi K, Otani S, Yamamoto H, et al. Pulmonary aspergillosis as a late complication after surgery for locally advanced non-small cell lung cancer treated with induction chemoradiotherapy. Surg Today. 2020;50:863–71. https://doi.org/10.1007/s00595-020-01960-5.
11. Carpagnano GE, Lacedonia D, Palladino GP, Logrieco G, Crisetti E, Susca A, et al. Aspergillus spp. colonization in exhaled breath condensate of lung cancer patients from Puglia Region of Italy. BMC Pulm Med. 2014;14:22. https://doi.org/10.1186/1471-2466-14-22.
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
13. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
14. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. https://doi.org/10.1002/jrsm.1411.
15. Tamura A, Suzuki J, Fukami T, Matsui H, Akagawa S, Ohta K, et al. Chronic pulmonary aspergillosis as a sequel to lobectomy for lung cancer. Interact Cardiovasc Thorac Surg. 2015;21:650–6. https://doi.org/10.1093/icvts/ivv239.
16. Whittaker G, Taylor M, Chamula M, Granato F, Balata H, Kosmidis C. Chronic Pulmonary Aspergillosis after Surgical Treatment for Non-Small Cell Lung Cancer—An Analysis of Risk Factors and Clinical Outcomes. J Fungi. 2024;10:335. https://doi.org/10.3390/jof10050335.
17. Kim B-G, Choi YS, Shin SH, Lee K, Um S-W, Kim H, et al. Mortality and lung function decline in patients who develop chronic pulmonary aspergillosis after lung cancer surgery. BMC Pulm Med. 2022;22:436. https://doi.org/10.1186/s12890-022-02253-y.
18. Zaini J, Al Maududi AA, Fillahihasanah T, Fadhillah MR, Pradono P, Haryanto B, et al. Pneumocystis jirovecii colonization in bronchoalveolar lavage among naïve non-small cell lung cancer from tertiary respiratory hospital in Jakarta, Indonesia. J Infect Dev Ctries. 2022;16:1643–7. https://doi.org/10.3855/jidc.15840.
19. Shin SH, Kim BG, Kang J, Um SW, Kim H, Kim HK, et al. Incidence and risk factors of chronic pulmonary aspergillosis development during long-term follow-up after lung cancer surgery. J Fungi. 2020;6:1–10. https://doi.org/10.3390/jof6040271.
20. Lee EH, Kim EY, Lee SH, Roh YH, Leem AY, Song JH, et al. Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci Rep. 2019;9:2094. https://doi.org/10.1038/s41598-019-38618-3.
21. Nishigaki Y, Fujita Y, Fujiuchi S, Hiramatsu M, Yamamoto Y, Takeda A, et al. Clinical aspects of pneumocystis pneumonia in patients with lung cancer. Japanese J Lung Cancer. 2009;49:241–7. https://doi.org/10.2482/haigan.49.241.
22. Shehbaz M, Aslam S, Arslan M, Nizamuddin S, Ali S, Abbas S. Clinical Characteristics and Outcomes of Pneumocystis jirovecii Pneumonia in Cancer Patients From a Tertiary Care Hospital. Cureus. 2023;15:e51291. https://doi.org/10.7759/cureus.51291.
23. Evans TJ, Lawal AA, Kosmidis C, Denning DW. Chronic Pulmonary Aspergillosis: Clinical Presentation and Management. Semin Respir Crit Care Med. 2024;45:88–101. https://doi.org/10.1055/s-0043-1776914.
24. Abu Rous F, Singhi EK, Sridhar A, Faisal MS, Desai A. Lung Cancer Treatment Advances in 2022. Cancer Invest. 2023;41:12–24. https://doi.org/10.1080/07357907.2022.2119479.
25. Chen J, Chen J, Ding HY, Pan QS, Hong WD, Xu G, et al. Use of an artificial neural network to construct a model of predicting deep fungal infection in lung cancer patients. Asian Pacific J Cancer Prev. 2015;16:5095–9. https://doi.org/10.7314/APJCP.2015.16.12.5095.
26. Mori H, Ohno Y, Ito F, Endo J, Yanase K, Funaguchi N, et al. Polymerase chain reaction positivity of Pneumocystis jirovecii during primary lung cancer treatment. Jpn J Clin Oncol. 2010;40:658–62. https://doi.org/10.1093/jjco/hyq040.
27. Togashi Y, Masago K, Ito Y, Sakamori Y, Okuda C, Fukuhara A, et al. Pneumocystis jiroveci pneumonia and colonization in patients with advanced lung cancer. Oncol Lett. 2013;5:601–4. https://doi.org/10.3892/ol.2012.1052.
28. Zaini J, Al Maududi AA, Annisa Z, Siregar DG, Setianingrum F, Tugiran M, et al. Diversity of Fungal Colonization in Respiratory Tract of Naïve Lung Cancer and The Emergence of Voriconazole Resistant Aspergillus. HAYATI J Biosci. 2023;30:1139–48. https://doi.org/10.4308/hjb.30.6.1139-1148.
29. Zhu ZZ, Hao HX, Tao R, Zhang YW. Identification of prognostic factors of chronic pulmonary aspergillosis: a retrospective cohort of 106 patients. J Thorac Dis. 2024;16:7310–9. https://doi.org/10.21037/jtd-24-831.
30. Kimura Y, Sasabuchi Y, Jo T, Hashimoto Y, Kumazawa R, Ishimaru M, et al. Epidemiology of chronic pulmonary aspergillosis: A nationwide descriptive study. Respir Investig. 2024;62:1102–8. https://doi.org/10.1016/j.resinv.2024.09.015.
31. Maitre T, Cottenet J, Godet C, Roussot A, Carime NA, Ok V, et al. Chronic pulmonary aspergillosis: Prevalence, favouring pulmonary diseases and prognosis. Eur Respir J. 2021;58. https://doi.org/10.1183/13993003.03345-2020.
32. Aguilar-Company J, Martín MT, Goterris-Bonet L, Martinez-Marti A, Sampol J, Roldán E, et al. Chronic pulmonary aspergillosis in a tertiary care centre in Spain: A retrospective, observational study. Mycoses. 2019;62:765–72. https://doi.org/10.1111/myc.12950.
33. de Oliveira VF, Viana JA, Sawamura MVY, Magri ASGK, Benard G, Costa AN, et al. Challenges, Characteristics, and Outcomes of Chronic Pulmonary Aspergillosis: A 11-Year Experience in A Middle-Income Country. Mycopathologia. 2023;188:683–91. https://doi.org/10.1007/s11046-022-00676-z.
34. Nie W, Lu J, Qian J, Wang S-Y, Cheng L, Zheng L, et al. Obesity and survival in advanced non-small cell lung cancer patients treated with chemotherapy, immunotherapy, or chemoimmunotherapy: a multicenter cohort study. BMC Med. 2024;22:463. https://doi.org/10.1186/s12916-024-03688-2.
35. Toussie D, Ginocchio LA, Cooper BT, Azour L, Moore WH, Villasana-Gomez G, et al. Radiation Therapy for Lung Cancer: Imaging Appearances and Pitfalls. Clin Chest Med. 2024;45:339–56. https://doi.org/10.1016/j.ccm.2024.02.007.
36. Terrones-Campos C, Ledergerber B, Specht L, Vogelius IR, Helleberg M, Lundgren J. Risk of Bacterial, Viral, and Fungal Infections in Patients With Solid Malignant Tumors Treated With Curative Intent Radiation Therapy. Adv Radiat Oncol. 2022;7:100950. https://doi.org/10.1016/j.adro.2022.100950.
37. Choi Y, Noh JM, Shin SH, Lee K, Um SW, Kim H, et al. The Incidence and Risk Factors of Chronic Pulmonary Infection after Radiotherapy in Patients with Lung Cancer. Cancer Res Treat. 2023;55:804–13. https://doi.org/10.4143/crt.2022.1305.
38. Vinod SK, Hau E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology. 2020;25:61–71. https://doi.org/10.1111/resp.13870.
39. Liebling M, Rubio E, Ie S. Prophylaxis for Pneumocystis jiroveci pneumonia: Is it a necessity in pulmonary patients on high-dose, chronic corticosteroid therapy without AIDS? Expert Rev Respir Med. 2015;9:171–81. https://doi.org/10.1586/17476348.2015.1002471.
40. Kamel T, Janssen-Langenstein R, Quelven Q, Chelly J, Valette X, Le MP, et al. Pneumocystis pneumonia in intensive care: clinical spectrum, prophylaxis patterns, antibiotic treatment delay impact, and role of corticosteroids. A French multicentre prospective cohort study. Intensive Care Med. 2024;50:1228–39. https://doi.org/10.1007/s00134-024-07489-2.
41. Luque Paz D, Jouneau S, Tattevin P, Ricordel C. Pneumocystis in metastatic lung cancer, a pragmatic approach in support of prophylaxis. BMJ Case Rep. 2021;14:e232895. https://doi.org/10.1136/bcr-2019-232895.
42. Bai X, Wang H, Tang Y, Xiao C, Gao Y, Tong H, et al. Lung adenocarcinoma concurrent with pulmonary cryptococcosis: a case report and literature review. BMC Pulm Med. 2024;24:416. https://doi.org/10.1186/s12890-024-03242-z.
43. Ng KL, Huan N, Tan WL, Aminudin NHM, Hassan F, Nordin KM. Unravelling lung adenocarcinoma in an immunocompetent patient with endobronchial aspergilloma: A case report. Respirol Case Reports. 2024;12:e01409. https://doi.org/10.1002/rcr2.1409.
44. Setiwalidi K, Li Y, Ma Y, Hao Z, Zhao Y, Zhang Y, et al. Invasive aspergillosis complicated in a patient with non-small cell lung cancer harboring RET fusion during treatment with RET-TKIs: a case report and literature review. Front Oncol. 2024;14:1431908. https://doi.org/10.3389/fonc.2024.1431908.
45. Guziejko K, Klukowska K, Budzińska U, Mróz RM. Case Report: Chronic Pulmonary Aspergillosis—An Unusual Long-Term Complication of Lung Cancer Treatment. Front Med. 2022;8:777457. https://doi.org/10.3389/fmed.2021.777457.
46. Hiba Z, Abdelmoughit H, Zaynab IH, Hounaida J, Rachida L, Youssef O. Pneumocystis pneumonia in patient with lung adenocarcinoma: early side effects from pembrolizumab. Radiol Case Reports. 2022;17:3979–81. https://doi.org/10.1016/j.radcr.2022.07.083.
47. Kim T-W, Lee J-H, Lee H-J, Kim S-W, Choi H-S. Pneumocystis Pneumonia in a Non-Immunocompromised Lung Cancer Patient after Surgery: A Case Report. Healthcare. 2022;10:2063. https://doi.org/10.3390/healthcare10102063.
48. Doello K, Amezcua V, García J, Valdivia J. Pneumocystis jirovecii pneumonia in a non-small cell lung cancer patient on chemoradiotherapy: A case report. Saudi J Med Med Sci. 2020;8:53–5. https://doi.org/10.4103/sjmms.sjmms_255_18.
49. Yao K, Qiu X, Hu H, Han Y, Zhang W, Xia R, et al. Pulmonary cryptococcosis coexisting with central type lung cancer in an immuocompetent patient: A case report and literature review. BMC Pulm Med. 2020;20:161. https://doi.org/10.1186/s12890-020-01200-z.
50. Uehara Y, Kasai H, Nakajima T, Tanabe N, Tatsumi K, Yoshino I. Aspergillus sternomyelitis developed from chronic pulmonary aspergillosis as a late complication to lobectomy for lung cancer. Intern Med. 2018;57:2991–4. https://doi.org/10.2169/internalmedicine.0334-17.
51. Boyd M, Ojha S, Goyos J, Cragun WH, Rubio E. Invasive pulmonary aspergillosis and lung adenocarcinoma: Case report. Thorac Cancer. 2013;4:212–4. https://doi.org/10.1111/j.1759-7714.2012.00145.x.
52. Santos VM dos, Trindade MC da, Souza DW da S de, Menezes AIC de, Oguma PM, Nascimento ALO. A 76-year-old Man with a Right Lung Adenocarcinoma and Invasive Aspergillosis. Mycopathologia. 2013;176:113–8. https://doi.org/10.1007/s11046-013-9651-2.
53. Uchida Y, Tsukino M, Shigemori W, Hayashi E, Watanabe I, Nakayama T, et al. Diagnosis of pulmonary mucormycosis aiding the diagnosis of small cell lung cancer. J Med Microbiol. 2012;61:1610–3. https://doi.org/10.1099/jmm.0.040766-0.
54. Neuville M, Borie R, Rodier JM, Debray MP, Danel C, Dombret MC, et al. Pneumocystose chez un patient traité par pemetrexed pour adénocarcinome pulmonaire. Rev Mal Respir. 2011;28:97–100. https://doi.org/10.1016/j.rmr.2010.06.029.
55. Velcheti V, Govindan R. Pneumocystis pneumonia in a patient with non-small cell lung cancer (NSCLC) treated with pemetrexed containing regimen. Lung Cancer. 2007;57:240–2. https://doi.org/10.1016/j.lungcan.2007.02.010.
56. Itano H, Andou A, Date H, Shimizu N. Non-small cell lung cancer coexisting with pulmonary aspergilloma. Japanese J Thorac Cardiovasc Surg. 2005;53:513–6. https://doi.org/10.1007/s11748-005-0099-2.
57. Tashiro M, Takazono T, Izumikawa K. Chronic pulmonary aspergillosis: comprehensive insights into epidemiology, treatment, and unresolved challenges. Ther Adv Infect Dis. 2024;11:20499361241253751. https://doi.org/10.1177/20499361241253751.
58. Rhoads S, Maloney J, Mantha A, Van Hook R, Henao-Martinez AF. Pneumocystis jirovecii Pneumonia in HIV-Negative, Non-transplant Patients: Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr Fungal Infect Rep. 2024;18:125-35. https://doi.org/10.1007/s12281-024-00482-8.
59. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785-94. https://doi.org/10.1111/biom.12817.
60. Classen AY, Henze L, von Lilienfeld-Toal M, Maschmeyer G, Sandherr M, Graeff LD, et al. Primary prophylaxis of bacterial infections Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO). Ann Hematol. 2021;100(6):1603-20. https://doi.org/10.1007/s00277-021-04452-9.
61. Park JW, Curtis JR, Choi SR, Kim MJ, Ha YJ, Kang EH, et al. Risk-Benefit Analysis of Pri-mary Prophylaxis Against Pneumocystis Jirovecii Pneumonia in Patients With Rheu-matic Diseases Receiving Rituximab. Arthritis Rheumatol. 2023;75(11):2036-44. https://doi.org/10.1002/art.42541.



 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Pocket
Pocket